Everything You Were Taught About Osmosis Is Wrong.
Osmosis is the reason that a fresh water fish placed in the ocean desiccates and dies. Osmosis is the reason that blisters form on fiberglass boat hulls. Osmosis is how waste products of metabolism enter and leave the blood stream. Osmosis determines how you, me and every living thing lives and dies. One would think that a civilization that spends billions of dollars every year on medical research would understand something as basic as osmosis. Wrong, wrong, wrong.
Osmosis is the tendency of water in salt water to
flow from an area of low salt concentration to an area of high salt
concentration across a semi permeable membrane. Of
course, osmosis applies to all solvents, not just water, and to all
solutes, not just salt. I will discuss only water and salt for
simplicity. Salt in solution comprises ions of chlorine
and sodium, but I will refer to 'dissolved salt' for simplicity.
A semi permeable membrane is a barrier that has holes large enough
to allow molecules of water to pass but small enough to block the
passage of the dissolved salt. In the example of the fresh
water fish placed in the ocean, the water in the fish has a
lower salt concentration than the surrounding ocean. The water
in the fish moves through the semi permeable membrane (the cells of
the gills of the living fish) from the area of low salt concentration (the fish)
to the area of high salt concentration (the ocean). As a
result, the fish dies of dehydration while surrounded by water.
surrounding ocean. The water
in the fish moves through the semi permeable membrane (the cells of
the gills of the living fish) from the area of low salt concentration (the fish)
to the area of high salt concentration (the ocean). As a
result, the fish dies of dehydration while surrounded by water.
But how does this process actually work? The
ions making up the dissolved salt in the salt water jiggle at random
due to Brownian motion. The ions bounce against all of the
boundaries of the salt water, including the cells of the gills of
the unfortunate fish. Because the salt concentration is higher
outside the cells than inside the cells, more jiggling ions bounce
against the outside of the cell walls than the inside of the cell
walls. The net force on the outside of the cell walls caused
by impacts of the jiggling ions squeezes the water from the cells.
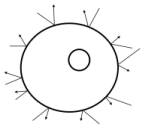
The image at right represents a cell of the fish's gill. The arrows represent impacts of the dissolved ions against the outside of the cell wall, sqeezing the cell. There are ions on the inside of the cell as well, but there are more ions, and hence a higher net force, on the outside due to the higher salt concentration.
How about the familiar demonstration of osmosis;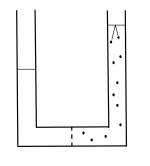 namely, a U-shaped tube contains vertical columns of water on either
side with a semi-permeable membrane in the middle? One of the
columns of water contains dissolved salt and and the other column of
water does not contain the dissolved salt. In the familiar
demonstration, the levels of water in the two columns start out
equal. Water then flows through the semi-permeable membrane so
that the water level in the low-concentration column falls and the
level in the high-concentration column rises.
namely, a U-shaped tube contains vertical columns of water on either
side with a semi-permeable membrane in the middle? One of the
columns of water contains dissolved salt and and the other column of
water does not contain the dissolved salt. In the familiar
demonstration, the levels of water in the two columns start out
equal. Water then flows through the semi-permeable membrane so
that the water level in the low-concentration column falls and the
level in the high-concentration column rises.
How does this demonstration work? The ions of the dissolved salt, indicated by dots in the drawing above, move in all directions due to Brownian motion. The ions bounce against each other and against all of the boundaries of the salt water, including the free surface. The free surface is where the salt water meets the air. When the ions making up the salt bounce against the free surface, the jiggling ions press against the liquid water molecules at the free surface. The liquid water molecules at the free surface are bonded to all of the other liquid water molecules and pull on all of the liquid water molecules, including the pure water on the other side of the semi permeable membrane. The pressure exerted by the ions making up the salt bouncing against the free surface pulls water through the semi permeable membrane.
HULETT'S EXPLANATION
G. Hulett outlined the above explanation of osmosis about a hundred years ago. It is simple, elegant, fully describes reality, and is all but forgotten today.
LEWIS' EXPLANATION
Five years later,
G.N. Lewis, one of the
"Founders of Thermodynamics," launched a different and ultimately
incorrect theory of osmosis. He theorized
that salt in water alters
the basic characteristics of the water, allowing pure water with
characteristics not altered by the salt to push through the
membrane. Lewis invented the terms "fugacity" and "activity"
to describe the characteristics of the salt water that he believed
were altered. Lewis did not explain the mechanism by
which the salt altered the fugacity and activity of the salt water.
Instead, he believed that fugacity and activity were simply physical
characteristics, like mass and volume.
Lewis' explanation was widely adopted and values for fugacity and activity were developed empirically to allow engineers to design for osmosis. The fugacity and activity values are useful in the same way that the steam tables are useful - they provide an algorithm to allow industrial designers to design machines and processes. The tables of fugacity and activity are empirically derived and have no theoretical foundation. Lewis' theory adds nothing to the understanding of osmosis.
Unfortunately, if you studied osmosis, you probably were taught Lewis' incorrect theory.
H. T. HAMMEL, Ph.D.
 The late Harold T. Hammel, Ph.D., had a long and
distinguished career. At the time of his passing in 2005, he
was a physiologist and emeritus professor of physiology at the
University of California, San Diego and adjunct professor of
physiology and biophysics at Indiana University. Dr. Hammel
proved that
Hullett was right and
Lewis was wrong. He also extended Hullett's theory of
osmosis to address the dynamic situation of living tissues of the
human body and of diffusing solutes in
moving solutions and changing concentrations of
solutes, such as blood plasma. In a concrete application of his
ideas, Dr. Hammel developed the first coherent explanation of high
altitude pulmonary edema ("HAPE") at the molecular level.
I prepared a
patent application to address
Dr. Hammel's understanding of HAPE. The purpose of the patent
application is to provide a physical application of Dr. Hammel's
ideas and to bring those ideas to the attention of engineers,
biochemists, physicians and technologists who might not be exposed
to
Dr. Hammel's articles
in scholarly journals.
The late Harold T. Hammel, Ph.D., had a long and
distinguished career. At the time of his passing in 2005, he
was a physiologist and emeritus professor of physiology at the
University of California, San Diego and adjunct professor of
physiology and biophysics at Indiana University. Dr. Hammel
proved that
Hullett was right and
Lewis was wrong. He also extended Hullett's theory of
osmosis to address the dynamic situation of living tissues of the
human body and of diffusing solutes in
moving solutions and changing concentrations of
solutes, such as blood plasma. In a concrete application of his
ideas, Dr. Hammel developed the first coherent explanation of high
altitude pulmonary edema ("HAPE") at the molecular level.
I prepared a
patent application to address
Dr. Hammel's understanding of HAPE. The purpose of the patent
application is to provide a physical application of Dr. Hammel's
ideas and to bring those ideas to the attention of engineers,
biochemists, physicians and technologists who might not be exposed
to
Dr. Hammel's articles
in scholarly journals.
Dr. Hammel felt so strongly about his discoveries that he had a summary engraved on his headstone. The text reads: "A physiologist who measured xylem and phloem sap pressures in trees, who embraced Hulett's theory of osmosis and who recognized the diffusion of bicarbonate ions as the principal osmotic effect in Starling's hypothesis."
MORE ABOUT OSMOSIS
Want to know more? For a succinct and rigorous explanation of osmosis, follow this link.

